n3- protons neutrons electrons|4.4: Protons, Neutrons, and Electrons : Baguio The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons . Converting EDT to CDT. This time zone converter lets you visually and very quickly convert EDT to CDT and vice-versa. Simply mouse over the colored hour-tiles and glance at the hours selected by the column. and done! EDT stands for Eastern Daylight Time. CDT is known as Central Daylight Time. CDT is 1 hours behind EDT.
PH0 · Protons, Neutrons, and Electrons
PH1 · Number of Protons, Neutrons, and Electrons in an Atom
PH2 · Number of Protons, Neutrons, and Electrons in an
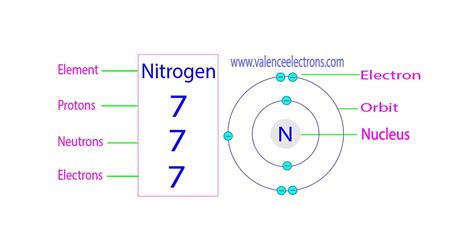
PH3 · Nitrogen
PH4 · How to find the number of protons, neutron, and electrons.
PH5 · How to find Protons & Electrons for the Nitride ion (N 3
PH6 · How to Find the Number of Protons, Neutrons, and Electrons
PH7 · How to Find the Number of Protons, Neutrons, and
PH8 · Atom Calculator
PH9 · 4.4: Protons, Neutrons, and Electrons
PH10 · 3.2.6: Subatomic Particles
PH11 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH12 · 1.8: Subatomic Particles
Philippine Merchant Marine School is a College in the Philippines. Visit CourseFinder.ph to see tuition fee and available courses as well as inquire for more information and enroll in a course. Home; About; . Philippine Merchant Marine School (PMMS) is a coeducational, nautical science institution founded on August 1, 1950 by a group of .
n3- protons neutrons electrons*******Electrons are one of three main types of particles that make up atoms. Unlike protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that do not consist of smaller particles. They are a type of fundamental particle called leptons. All leptons have . Tingnan ang higit pa
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the . Tingnan ang higit paAtoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically charged, . Tingnan ang higit pa
The number of protons in the nucleus of an atom is its atomic number (Z Z). This is the defining trait of an element: Its value determines the identity . Tingnan ang higit pa
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons . Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons .
Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron). The . Figure 3.2.6.2 3.2.6. 2: Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, . Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Key Takeaways: Number of Protons, Neutrons, and Electrons. Atoms are made of . Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, making up the nucleus. .
In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Nitride ion (N3-).
Atoms are made of three kinds of particles: neutrons, protons, and electrons. Protons and neutrons form the nucleus of the atom , and electrons .
4.4: Protons, Neutrons, and Electrons The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The . The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (Figure \(\PageIndex{1}\)). These .
The diameter of an atom of hydrogen is 0.1nm (1.0nm = 10-9 m). So, if 1000 crore atoms of hydrogen are arranged side by side, it will be 1 meter long. However, it has been possible to detect atoms by increasing the vision of a very powerful electron microscope by two million times.
Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported .
Les trois parties d'un atome sont des protons chargés positivement, des électrons chargés négativement et des neutrons neutres. Suivez ces étapes simples pour trouver le nombre de protons, de neutrons et d'électrons pour un . Solution: The notation e-refers to electrons and p + refers to protons. The number of protons is an element's atomic number. Use the periodic table to find the element with an atomic number of 7. This element is nitrogen, which has the symbol N. The problem states that there are more electrons than protons, so we know the ion has a .A nitride ion has 7 protons, 8 neutrons, and 10 electrons. What is the overall charge on this ion? Q. A charged body has 7 electrons and 10 protons. It can be made neutral by adding: . How many protons and electrons are present in C a 2 + ion ? Q. Compare the radii of two species X and Y. (a) X has 12 protons and 12 electrons. (b) Y has 12 .

O cálculo do número de partículas atômicas é utilizado para indicar a quantidade de prótons (no núcleo), elétrons (na eletrosfera) e nêutrons (no núcleo) presentes em um átomo ou íon .
Study with Quizlet and memorize flashcards containing terms like Specify the number of protons, neutrons, and electrons in the neutral atom bromine-80., The ion N3− has _____ protons and _____ electrons., What isotope has 17 protons and 18 neutrons? and more.
Protons, Neutrons and Electrons: The number of protons in an atom uniquely determines the identity of the element. This number is called the atomic number. If the atom is electrically neutral, then the number of electrons is also equal to the number of protons. . How many protons and electrons are in the ion N3-? How many protons and . The nitride ion, "N"^(3-) has 7 protons and 10 electrons. The formula of the nitride ion, "N"^(3-), indicates that a nitrogen atom has gained 3 additional electrons. Nitrogen has atomic number 7, which means its atoms have 7 protons in their nuclei. In a neutral nitrogen atom, there would also be 7 electrons. But since the nitride ion has a 3^ .Figure \(\PageIndex{1}\): Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball! Protons. A proton is another one of three main particles that make up the atom. Protons are found in the nucleus of the atom – the tiny, extremely dense . In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Phosphide ion (P3-). From the Periodic . Table \(\PageIndex{1}\) gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (\(\text{amu}\)) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units (\(\text{amu}\)) are useful, .
Comme les protons et les neutrons sont dans le noyau, on les appelle aussi les NUCLÉONS (noyau et nucléon sont deux mots ayant la même racine). Donc quand on parle de nucléons, on parle des protons et des neutrons sans distinction. Un nucléon peut donc être un proton ou un neutron. Masse des protons, neutrons, et .Atomic Number – Protons, Electrons and Neutrons in Nitrogen. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e .
Pour trouver le nombre de neutrons, il faut arrondir la masse atomique à l’unité, ce qui équivaut au nombre de masse, et en soustraire le numéro atomique, qui correspond au nombre de protons. On écrit ensuite le nombre de neutrons dans le cercle, accompagné de « n 0 », qui désigne le mot neutron.
The number of protons in an atom. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. . This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average.
n3- protons neutrons electronsThe mass of an electron is very small compared to a proton or a neutron. 1 840 electrons have the same mass as 1 proton or neutron. Since the nucleus only contains protons and neutrons, most of .
FIND OUT MORE ABOUT THIS GAME ON THE SITEhttps://ultimatehistoryvideogames.jimdo.com/i-robotChronological playlist for all games .
n3- protons neutrons electrons|4.4: Protons, Neutrons, and Electrons